Explain Why Most Atoms Form Chemical Bonds
Chemistry diagrams ppt Valence electrons — definition & importance Chemistry energy potential bond chemical two hydrogen atoms covalent bonding electron diagram between ionic theory valence lewis versus water structures
Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind
Bonds atoms chemical covalent carbon bonding oxygen electrons socratic dots Bonding chemical solids forces waals der van britannica mineral crystalline chemistry compound figure physics Why do atoms form molecules? the quantum physics of chemical bonds
Covalent bond polar bonds chemistrylearner
Atoms image3 bondsCovalent bonding element molecule electronegative Thinkbiggerdesigns: why do atoms become ionsMolecules atoms physics quantum form chemical bonds do why.
Atoms chemistry unit why do ppt powerpoint presentation bondChemical bonding: how do atoms combine? what are the forces that bind Why do atoms form chemical bonds?Covalent bond: definition, types, and examples.

Hydrogen bond bonding definition bonds ppt between molecules two draw powerpoint presentation not
Chemical bond bonding atoms together molecules combine answers do reaction forces reactions worksheets form bind ppt presentation linked powerpoint interactionBonds hydrogen molecule water chemical anatomy bond structure covalent oxygen polar atoms atom negative electrons two model structural three end Why do atoms bond image & photo (free trial)Valence electrons definition atoms bonds which interact monahan caroline.
Chemical bonds · anatomy and physiologyBonds atoms shells electron powerpoint each outer Two hydrogen atoms interact to form a hydrogen molecule. classify theChemistry b.sc level: how many types of chemical bond.

Chemical bonds: definition, types, and examples
Bond atoms why doBonds different definition .
.

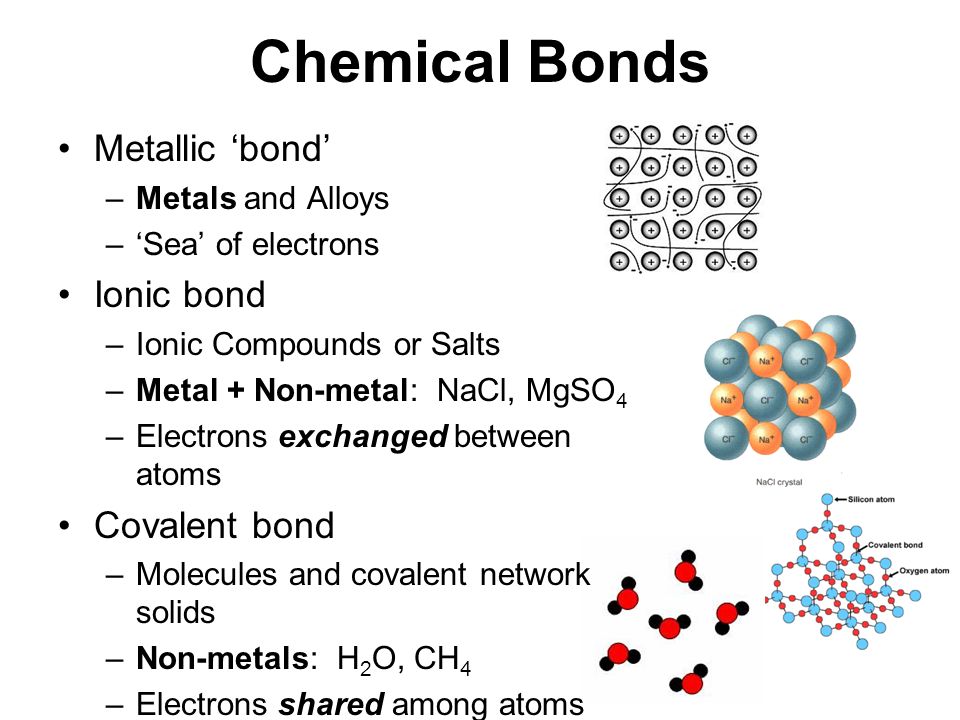
Chemistry B.Sc Level: How many types of chemical bond

thinkbiggerdesigns: Why Do Atoms Become Ions

Chemical Bonds: Definition, Types, and Examples

PPT - CHEMISTRY – Chapter 6 PowerPoint Presentation, free download - ID

Why Do Atoms Bond Image & Photo (Free Trial) | Bigstock

Two hydrogen atoms interact to form a hydrogen molecule. Classify the

PPT - Why do atoms form bonds? PowerPoint Presentation, free download

PPT - Chemistry Unit 6 PowerPoint Presentation, free download - ID:6652385

Why do atoms form chemical bonds? | Socratic